News, Publications
Toward an International Standardisation Roadmap for Nanomedicine

A Key Step Forward in the MetrINo Project
A recent publication in Drug Delivery and Translational Research by Caputo, F. et al. (2024), titled “Toward an International Standardisation Roadmap for Nanomedicine“, highlights the urgent need for harmonised test methods and reference materials to support the clinical translation of nanomedicines. This work aligns closely with the objectives of the MetrINo project, which aims to establish robust metrological frameworks for nanotherapeutics.

Key Takeaways from the Study
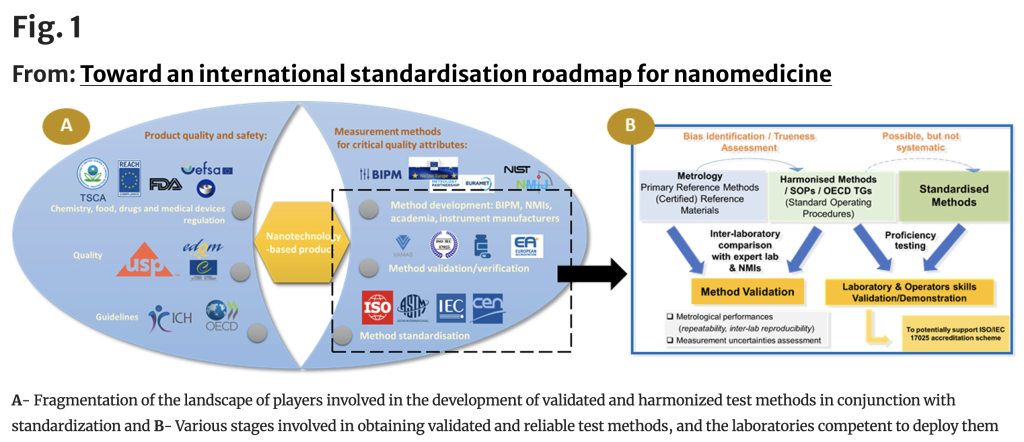
- Standardisation as a Priority: The study underscores the lack of harmonised analytical methods and reference materials as a major barrier to the regulatory acceptance of nanomedicines. Global
- Collaboration: The findings stem from a high-level workshop held in Paris in October 2023, gathering experts from metrology institutes, regulatory agencies, industry, and academic research. Participants agreed on the need for a unified approach to standardisation, involving international organisations such as ISO, CEN, ASTM, VAMAS, and regulatory bodies.
- Focus on High-Priority Nanomedicines: The paper identifies lipid nanoparticles (LNPs) and liposomal formulations—widely used in mRNA vaccines and drug delivery—as key targets for standardisation efforts, followed by metal oxide nanoparticles for diagnostics and radiotherapy.
- Next Steps: One major outcome is the proposal to establish a dedicated Working Group within CEN/TC 352 to structure the European standardisation efforts in nanomedicine, ensuring alignment with global regulatory expectations.
MetrINo’s Contribution
As part of its mission, MetrINo is directly contributing to this roadmap, supporting the development of standard operating procedures (SOPs) and new reference materials (RMs) for the nanomedicine community. The project’s expertise in metrology and characterisation methods will be instrumental in overcoming these regulatory challenges.
This publication strengthens MetrINo’s role in fostering a coordinated, international standardisation strategy for nanomedicine. It also aligns with our broader goal of enabling faster and safer clinical translation of nanomedicine innovations.
Read the full article: Caputo, F., Favre, G., Borchard, G., Calzolai, L., Fisicaro, P., Frejafon, E., Günday-Türeli, N., Koltsov, D., Minelli, C., Nelson, B. C., Parot, J., Prina-Mello, A., Zou, S., & Ouf, F. X. (2024). Toward an international standardisation roadmap for nanomedicine. Drug Delivery and Translational Research, 14(9), 2578–2588..
🔗 Available in open access here: Springer Link – DOI: 10.1007/s13346-024-01646-2