News
Cutting-Edge Cryo-Characterisation for Lipid Nanoparticles
Discover how advanced surface chemical analysis techniques are revolutionizing the development and characterization of lipid nanoparticles (LNPs) in nanomedicine. The METRINO project is pioneering innovative approaches to the use of these techniques to ensure precise and detailed surface chemical analysis, which provides deeper insights into the attributes enhancing the efficacy and stability of nanotherapeutics for various applications, from cancer treatment to advanced drug delivery systems.
Enhancing Healthcare with Nanomedicines
With the world’s first personalised mRNA vaccine against one of the most aggressive forms of skin cancer progressing to phase 3 clinical trials, nanoparticle-based complex therapeutics continue to deliver tangible strategies for tackling global challenges. This success builds on the same technology platform used by the Moderna and Pfizer-BioNTech COVID-19 vaccines, demonstrating the versatile applications of nanomedicines.Particle-based complex therapeutic platforms are used not just for the development of vaccines, but also as chemotherapeutics with reduced toxicity. These nanosystems rely on engineered particles that protect the therapeutic cargo and effectively deliver it into the body. Understanding and optimizing these delivery systems are paramount to their success. This brings us to the crucial role of surface characterization in optimizing these therapeutic nanoparticles.
The crucial role of Surface Characterisation in Nanomedicines
The surface of these particles is specifically designed to impart stability and sufficiently long circulation times to the systems. Current efforts focus on enhancing these surfaces with proteins that selectively target diseased cells, which can significantly increase efficacy and reduce toxicity, and thus step-change innovation in therapeutics.
Quantitatively describing and measuring the surfaces of these therapeutic nanoparticles is critical to optimize the design and development of these systems. Yet, the methods currently available to the pharmaceutical industry are indirect, rely on bulk analysis and lack spatial resolution.
Surface chemical methods such as X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) are considered the gold standard for the chemical analysis of the outermost materials’ surfaces, providing quantitative elemental composition and molecular make up. They hold then great potential for advancing the development of nanomedicines with engineered functional surfaces. In recent years these methods have been applied to a variety of materials and products, including nanoparticles [1-4]. The combination of these methods with gas-ion-cluster sputtering enables controlled milling and three-dimensional chemical mapping.
The Challenge
Traditional ToF-SIMS and XPS methods require for the sample to be placed in a high vacuum chamber during the analysis. However, this condition is incompatible with many nanomedicine systems, including liposomes, lipid nanoparticles, and certain polymer-based particles, as they do not maintain their structure in vacuum. So, while the chemical analysis of their components may be possible by ToF-SIMS and XPS, mapping their distribution within the products is challenging. Addressing these challenges is essential for advancing nanomedicine applications. METRINO’s innovative approaches offer potential solutions.
METRINO’s solutions
Innovative Cryo-Characterisation Techniques
The METRINO project is exploring different approaches to enable the challenging application of state-of-the-art XPS and ToF-SIMS methods to the analysis of these materials. One such strategy consists in depositing a drop of the products on a suitable substrate and rapidly freezing it to liquid-nitrogen temperatures before analysis. This process forms amorphous ice, preventing the growth of ice crystals that would disrupt the particle structure due to an increase in volume.
How unique is it?
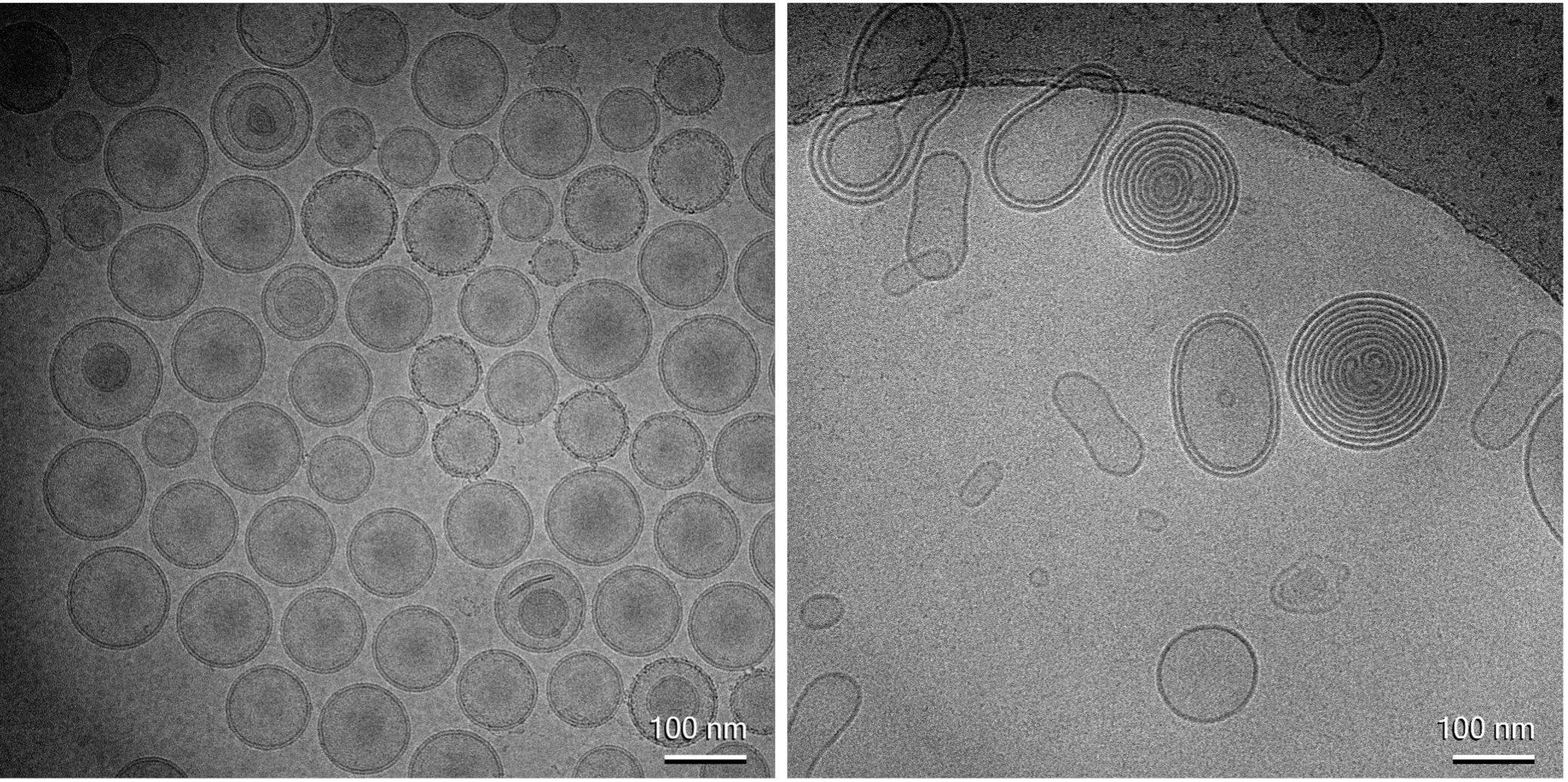
This approach is already successful in transmission electron microscopy (TEM) for these materials. Within the METRINO project, we use cryogenic TEM to visualize the materials and demonstrate that the cryogenic preparation process effectively preserves their structure. Cryogenic TEM imaging, as shown in Figure 1, provides detailed structural and dimensional information, enabling evaluation of the product’s homogeneity and structural integrity and purity.
Advancements in ToF-SIMS
The imaging of the materials is also incredibly valuable to guide the interpretation of the chemical analysis performed by XPS and ToF-SIMS.
In ToF-SIMS, mass spectra of surface molecular species are generated at sub-micron spatial resolution, allowing the mapping of specific molecules on the sample surface and measurements of the molecular composition of surface structures down to the sub-µm size range.
METRINO is developing this method to map individual liposomes and lipid nanoparticles (LNPs) in their native water matrix, aiming to probe the lipid composition of individual liposomes/LNPs.
Sample preparation involves placing a droplet of liposome solution between two mica sheets, followed by shock-freezing of this ‘sandwich’ sample in liquid nitrogen. Immediately before introduction into the ToF-SIMS instrument, the top mica sheet is removed, keeping the sample cold (T < -100 °C) during the entire transfer and analysis process (Figure 2a); this ensures the preservation of vitreous ice and avoids the formation of crystalline ice. Figures 2b show ion images of the liposome-containing ice matrix obtained after milling away a few micrometers of the top surface to obtain a fresh surface representing the bulk ice matrix. The liposomes are represented by the signal intensity of hydrocarbon fragment ions (CxHy+), whereas the ice matrix is represented by water cluster ions (H(H2O)+). The images clearly demonstrate the possibility to characterize individual liposomes (about 100 to 300 nm diameter) using this method.
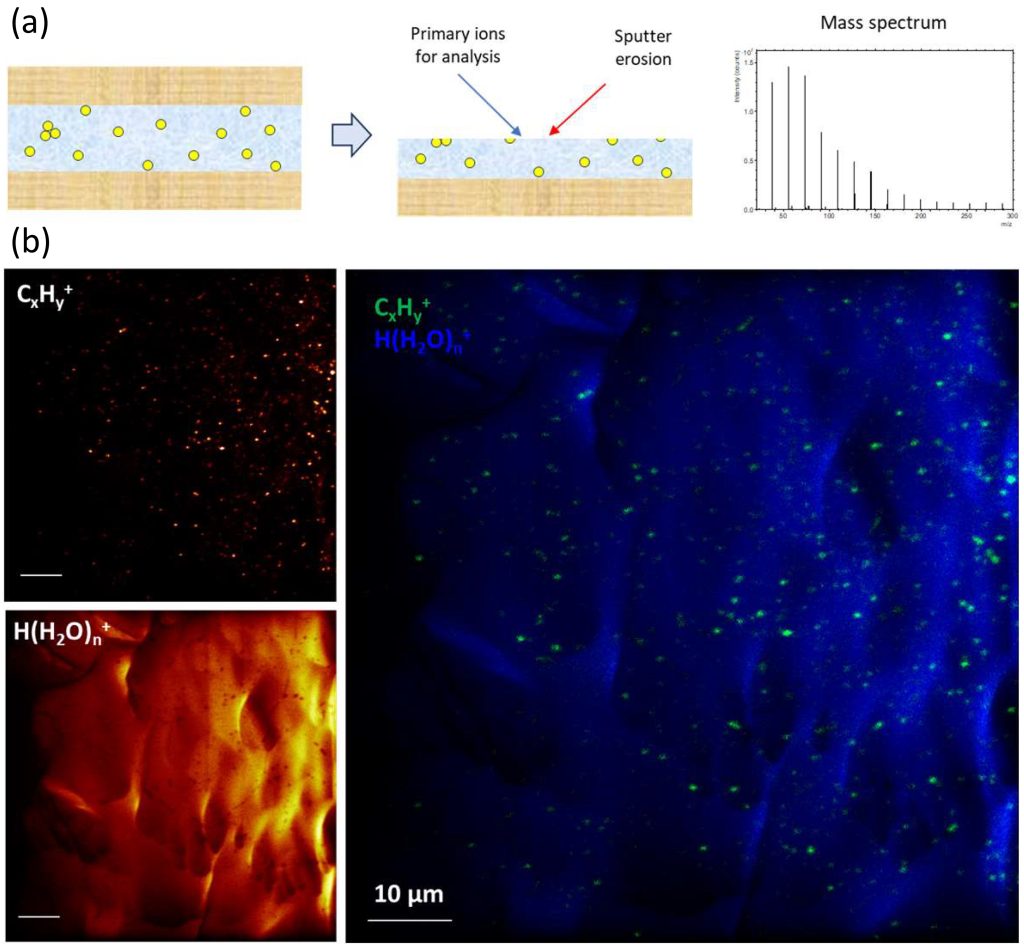
These results are very encouraging and ongoing work aims to extract information on lipid composition from mass spectra of individual liposomes. The potential outcome of this work is highly significant, because while the average bulk composition of liposomes or lipid nanoparticles can be determined with techniques like chromatography, the compositional variation between individual particles is largely unknown. These advancements in ToF-SIMS mark a significant step forward. Complementing these efforts, cryogenic XPS further enhances our ability to analyze and understand these complex systems.
Advancements in Cryogenic XPS
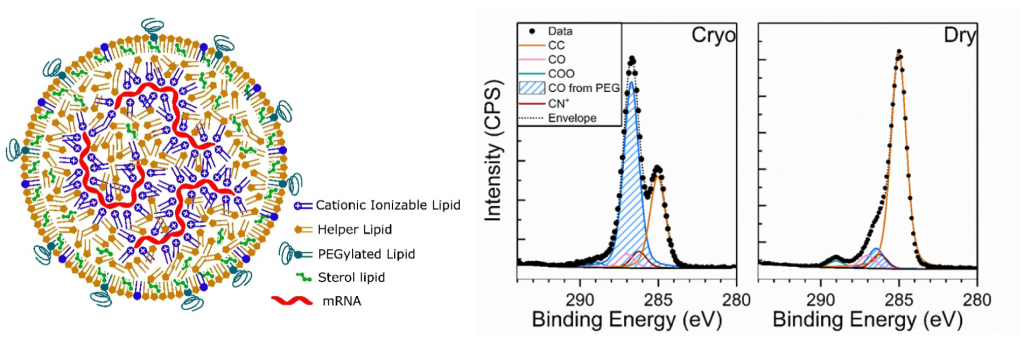
XPS has been successfully applied to nanomedicine analysis and continues to be advanced within the METRINO project. Although XPS is not suited for single particle resolution, it provides quantitative information on the average chemical composition of the particles. Figure 3 shows an example of the application of cryogenic XPS to the surface chemical analysis of RNA-loaded lipid nanoparticles manufactured by MetrINo partner SINTEF (Norway). The figure highlights significant differences between spectra measured under cryogenic conditions and at room temperature, with signal from the polyethylene glycol (PEG, highlighted in blue in the figure) layer enhanced in the former. Because XPS signal typically relates to the top 10 nm of organic materials, and since the PEG layer is expected to be located at the outermost interface of the particles (see the schematics), this result suggest a correlation of the signal measured by XPS to the structure of the sample prepared in cryogenic conditions.
Conclusion
Key take-aways
- Unique Information: Combining cryogenic technology with surface chemical analysis methods provides unique insights into nanomedicine products that other methods cannot offer, such as information on the lipid composition of individual nanoparticles and surface coatings.
- Lipid Composition Analysis: Measuring lipid composition on a particle-by-particle basis offers crucial insights into sample homogeneity, correlating with overall product efficacy.
- Surface Quantification: Quantitative measurement of particle surfaces allows direct verification of synthetic strategies to enhance stability and targeting potential.
- MetrINo’s Role: The MetrINo project pioneers these approaches by creating a network platform where measurement specialists collaborate with industrial nanomedicine manufacturers and international experts.
Stay Tuned: All work packages (WPs) will progress on all three pillars of METRINO’s actions: Standard Operating Procedures (SOPs), Reference Materials, and Community Building!
We invite your feedback and participation. For more information or to get involved in advancing nanomedicine, please contact us.
Not an expert in the field? Find a simple summary below to help you understand our work!
Imagine trying to study particles so small that they’re invisible to the naked eye, ten thousand times smaller than a millimetre. These nanoparticles are used in advanced medicines to make treatments more effective and safer. But there’s a challenge: these particles can easily lose their shape when examined. To solve this problem, scientists use extreme cold, freezing the particles instantly with liquid nitrogen. This technique, called cryo-preparation, keeps the particles in their natural state. Using powerful tools, scientists can then examine the frozen particles in great detail. This helps them understand how the particles are made and how to improve them. The MetrINo project is leading these efforts in Europe, working with experts worldwide to develop better nanomedicines. Together, we’re making sure these new methods are accurate, trustable and helpful for creating new treatments.
References
1. Carlred, L.; Gunnarsson, A.; Solé-Domènech, S.; Johansson, B.; Vukojević, V.; Terenius, L.; Codita, A.; Winblad, B.; Schalling, M.; Höök, F.; Sjövall, P., Simultaneous Imaging of Amyloid-β and Lipids in Brain Tissue Using Antibody-Coupled Liposomes and Time-of-Flight Secondary Ion Mass Spectrometry. Journal of the American Chemical Society 2014, 136 (28), 9973-9981.
2. Korin, E.; Froumin, N.; Cohen, S., Surface Analysis of Nanocomplexes by X-ray Photoelectron Spectroscopy (XPS). ACS Biomaterials Science & Engineering 2017, 3 (6), 882-889.
3. Wei, X.-Q.; Ba, K., Construction a Long-Circulating Delivery System of Liposomal Curcumin by Coating Albumin. ACS Omega 2020, 5 (27), 16502-16509.
4. Yu, S.; Perálvarez-Marín, A.; Minelli, C.; Faraudo, J.; Roig, A.; Laromaine, A., Albumin-coated SPIONs: an experimental and theoretical evaluation of protein conformation, binding affinity and competition with serum proteins. Nanoscale 2016, 8 (30), 14393-14405.
5. Cant, D. J. H.; Pei, Y.; Shchukarev, A.; Ramstedt, M.; Marques, S. S.; Segundo, M. A.; Parot, J.; Molska, A.; Borgos, S. E.; Shard, A. G.; Minelli, C., Cryo-XPS for Surface Characterization of Nanomedicines. The Journal of Physical Chemistry A 2023, 127 (39), 8220-8227.
Authors
Caterina Minelli,1 Torben Nilsson Pingel,2 William Lee,1 Annika Altskär,2 Laurence Poul,3 Alexandre Ceccaldi,4 Peter Sjövall2
1 National Physical Laboratory, UK
2 RISE Research Institutes of Sweden, Sweden
3 Curadigm, France
4 European Technology Platform on Nanomedicine, Europe