News
Early Insights from the METRINO clinical Survey!

As the field of nanomedicine rapidly evolves, understanding the diverse needs and perspectives within the community is more crucial than ever. The METRINO project is dedicated to developing metrological tools that address key challenges in drug delivery, biodistribution, and clinical administration of nanoparticles. To align our efforts with real-world demands, we launched the Clinical Nanomedicine Survey. Today, we’re excited to share early insights from the first 22 respondents—and we invite more experts, especially clinicians, to ANSWER OUR QUICK QUESTIONNAIRE, join the conversation and help shape the future of nanomedicine.
Who’s Contributing So Far?
Our survey respondents represent a diverse group of professionals from 16 countries, predominantly across Europe, but also from the United States, India, and Pakistan. Most respondents (63.6%) are from academic research institutions, bringing deep expertise in nanotechnology, material science, and pharmaceutical development. These contributors are key drivers of preclinical nanomedicine, holding roles such as professors, researchers, and principal investigators.
However, there’s a clear gap in representation from clinical professionals. This presents a valuable opportunity: we’re seeking clinicians, regulatory experts, and industry leaders to contribute their insights, bridging the gap between preclinical research and the real-world clinical application of nanomedicine.
Key Findings from the Survey
Nanoparticle Types in Use and Future Interest
- Current Focus: The majority of respondents (77%) are working with lipid-based nanoparticles—unsurprising given their proven success in vaccine developments and drug delivery systems.
- Emerging Interest: There is growing curiosity about metal-based nanoparticles, particularly iron oxide and hafnium oxide, due to their unique potential in diagnostics and therapy.
Application Areas
- Dominant Field: Unsurprisingly, cancer therapy remains the primary application focus, reflecting the urgent need for innovative treatments in oncology.
- Rising Trends: There’s significant interest in RNA-based therapeutics and vaccines, echoing global developments in mRNA technology. This shift suggests a growing focus on personalized medicine and novel treatment modalities.
Administration Routes
- Common Practices: Intravenous (IV) administration is the most commonly used method, adopted by 65% of respondents.
- Exploring Alternatives: Several respondents are exploring localized injections and inhalation techniques, seeking to improve targeted delivery while minimizing systemic side effects.
Challenges in Detection and Dosing
- Detection Sensitivity: Respondents reported a wide range of detection sensitivities, from nanomolar to millimolar concentrations. Tracking intact nanoparticles in vivo remains a challenge, often complicated by the need to differentiate between the nanoparticle and its cargo.
- Dosing Variability: Dosing spans a broad range, from micrograms to milligrams per kilogram, and varies by nanoparticle type, administration route, and therapeutic application.
Tissue Targets for ADME Studies
- Primary Focus: The liver, spleen, and lungs are the top tissues of interest for absorption, distribution, metabolism, and excretion (ADME) studies.
- Model Limitations: Many respondents highlighted that current preclinical models (both in vitro and in vivo) do not fully capture the complexities of human biology, emphasizing the need for more predictive models.
The Missing Piece: Clinical Expertise
One of the most striking insights is the imbalance in clinical representation among the respondents. While we have a strong foundation of nanotechnology expertise and preclinical research, the lack of clinical input underscores the need for insights from those who work on the frontlines of patient care and navigate the regulatory landscape.
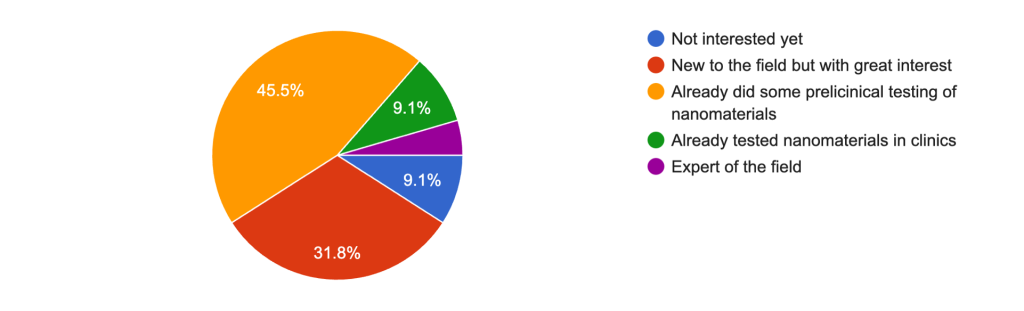
Why does this matter?
- Bridging the Gap: Bringing nanomedicine from the lab to the clinic requires a deep understanding of clinical challenges, patient needs, and regulatory hurdles.
- Enhancing Impact: Clinician insights can guide the development of metrological tools that are not only scientifically sound but also clinically applicable.
- Regulatory Alignment: Input from regulatory professionals will ensure our innovations meet the required safety and efficacy standards, accelerating the path to patient care.
An Invitation to Clinicians and Industry Experts
At METRINO, we’re committed to building solutions that resonate across the nanomedicine ecosystem—from researchers to patients. To achieve this, we’re reaching out to clinicians, regulatory authorities, and industry stakeholders to enrich our survey with their invaluable perspectives.
Your insights can drive change:
- Shape Metrological Tools: Help us develop measurement techniques that address real-world clinical challenges.
- Inform Best Practices: Contribute to the development of guidelines that enhance the reliability and reproducibility of nanomedicine research.
- Accelerate Innovation: Collaborate with us to identify and overcome barriers in clinical translation, ensuring effective nanomedicine therapies reach patients sooner.
Shaping the Future of Nanomedicine—Together
The preliminary findings from our survey offer a glimpse into the current landscape of nanomedicine research and highlight the need for a more comprehensive approach that includes clinical insights. By participating in the Clinical Nanomedicine Survey, you can play a pivotal role in steering the development of metrological tools that will shape the future of healthcare.
Key Takeaways for METRINO:

- Leverage Established Nanoparticles: Continue to build on the success of lipid-based nanoparticles while exploring the advantages of metal-based options.
- Align with Emerging Therapies: Focus on RNA-based therapeutics and vaccines, which are gaining global traction.
- Enhance Detection Methods: Develop sensitive and reliable metrological tools for accurately tracking nanoparticles in the body.
- Address Model Limitations: Invest in more predictive preclinical models to better emulate human physiology.
- Foster Multidisciplinary Collaboration: Actively seek partnerships with clinicians and regulatory experts to ensure our developments are clinically relevant and meet regulatory standards.
Let’s Connect and Collaborate
If you’re a clinician, regulatory professional, or industry expert in nanomedicine, we invite you to share your experiences and insights. Your contribution can make a significant impact in shaping the tools and methodologies that will drive nanomedicine forward.
Together, we can accelerate the journey from the laboratory to the clinic, improving patient outcomes and advancing global health.
Take the Survey Here and Join the Conversation!