News, Publications
Safer Metrology for Nanomedicine: EMPA’s Breakthrough in Analytical Methods
The EMPA laboratory, a key partner in the METRINO project, continues to advance the state-of-the-art in analytical methods for nanomedicine. Two recent publications from EMPA researchers highlight critical innovations designed to enhance safety, precision, and efficacy in nanoparticle characterization and electron microscopy, while significantly reducing laboratory hazards.
Eliminating Hazardous Hydrofluoric Acid in Metal Oxide Analysis
Group IV metal oxides (titanium, zirconium, and hafnium oxides) are extensively used in biomedical and industrial applications due to their stability and biocompatibility. However, their inert nature traditionally requires hydrofluoric acid (HF) for digestion—posing severe safety risks.
EMPA researchers developed an HF-free digestion method using sulfuric acid and hydrogen peroxide under microwave-assisted conditions. Their novel method achieved:
- Recoveries of over 90% across a variety of nanoparticle sizes and compositions.
- Successful validation in complex biological matrices, such as human cancer cells and bovine liver tissues, demonstrating the robustness and versatility of the method.
- Comparable sensitivity and detection limits to traditional HF-based methods, but with significantly reduced health and environmental risks.
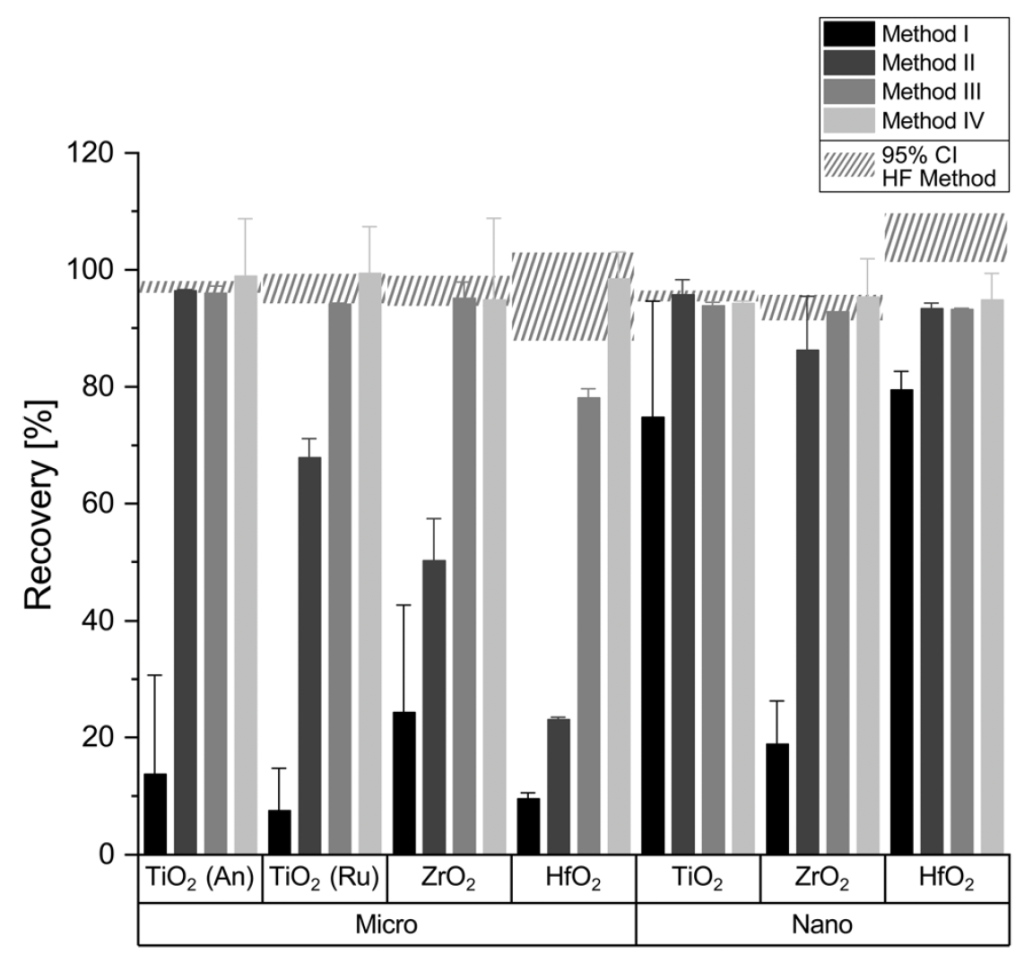
Comparison of recovery efficiencies using traditional HF digestion versus new HF-free methods: Metal oxide recoveries for micro- and nanopowders and four different digestion methods determined from measuring the single elements (Ti, Zr, Hf) and calculated using eqn (1); method I: standard HNO3, 10 min@200 °C, method II: H2SO4, 10 min@200 °C; method III: H2SO4, 30 min@250 °C, method 4: piranha, 30 min@250 °C; An: anatase, Ru: rutile. Error bars signify 2srel (∼95% confidence interval (CI)) and the hatched area the 95% CI of the HF reference method for direct comparison.
Read the full publication in Analytical Methods, Gerken et al., 2025.
Safe and Superior Alternatives to Uranyl-Based TEM Staining
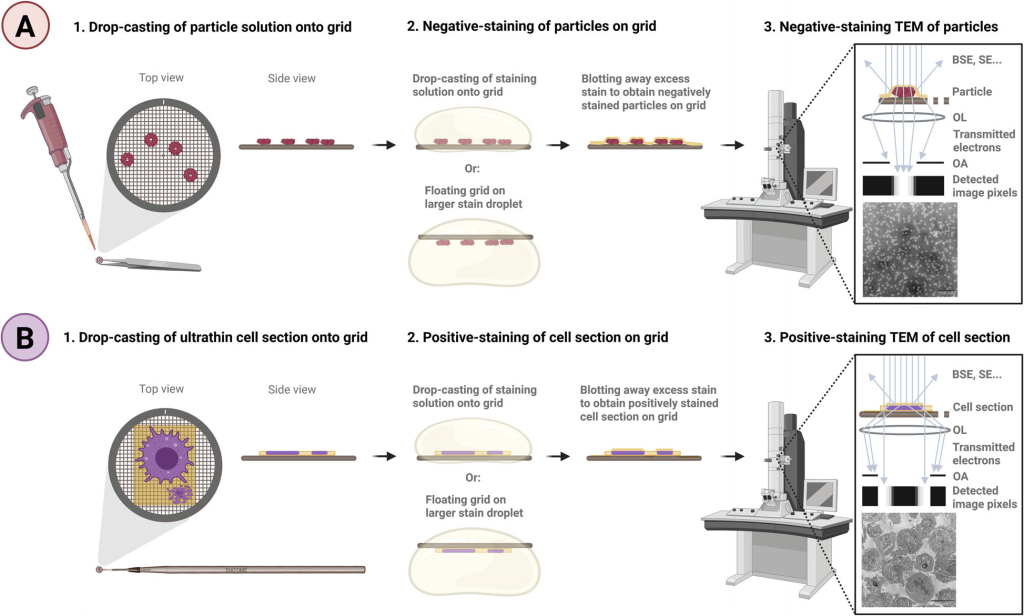
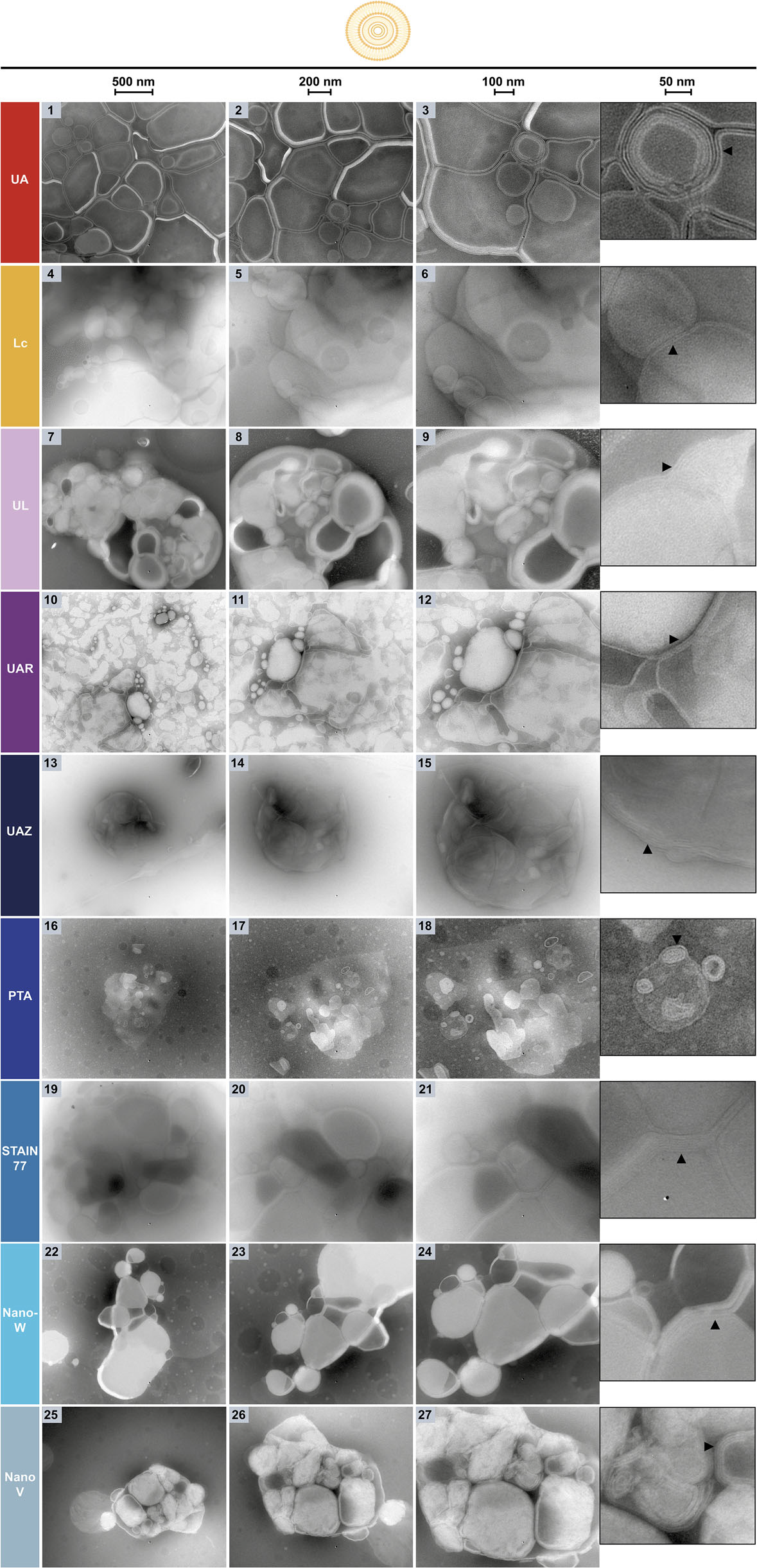
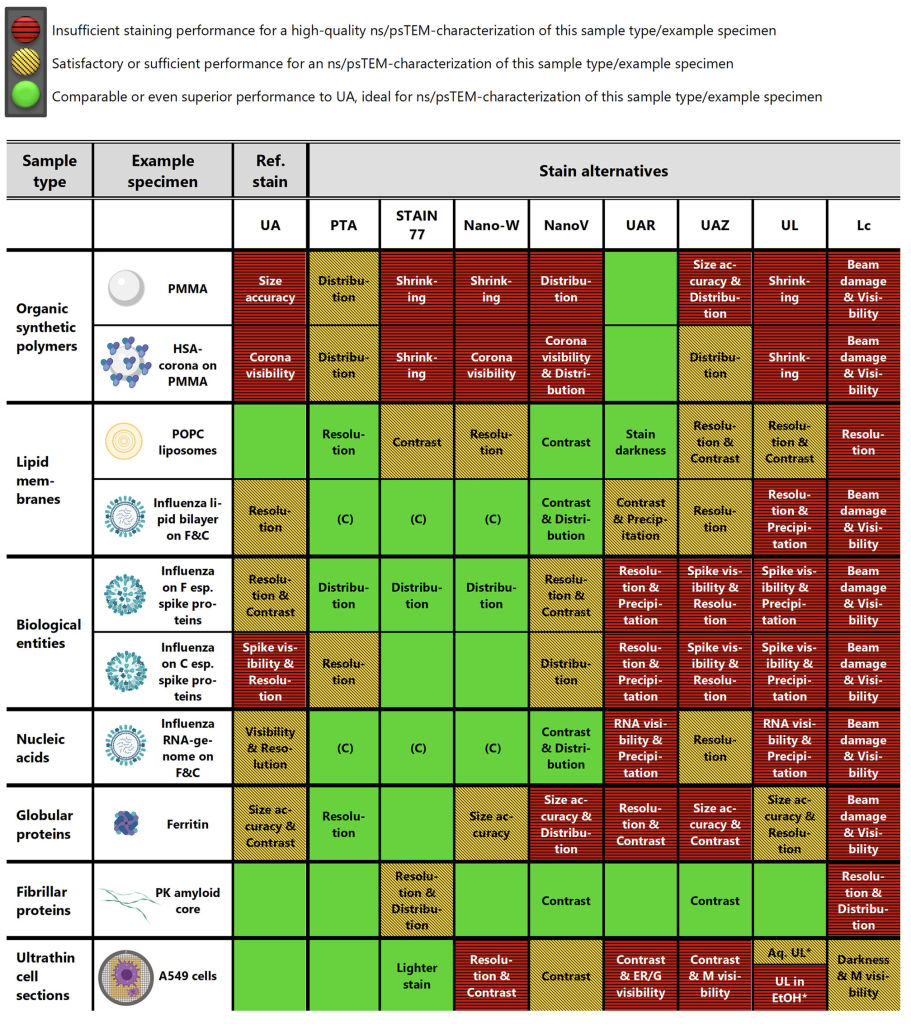
Negative & Positive Staining Transmission electron microscopy (TEM) typically relies on uranyl acetate (UA), a radioactive and toxic reagent. EMPA conducted a systematic evaluation of commercial uranyl-alternative stains, identifying safer and highly effective substitutes:
- Several commercial, uranyl-free stains (UAR, PTA, STAIN 77, and Nano-W) were found to achieve not only comparable but even superior staining performance to uranyl acetate, offering excellent visualization of organic samples ranging from nanoparticles to biological specimens
- A decision-making tool, GUIDE4U, was developed to easily select the optimal uranyl-replacement stains for a wide variety of organic specimens for TEM imaging and other EM applications requiring staining
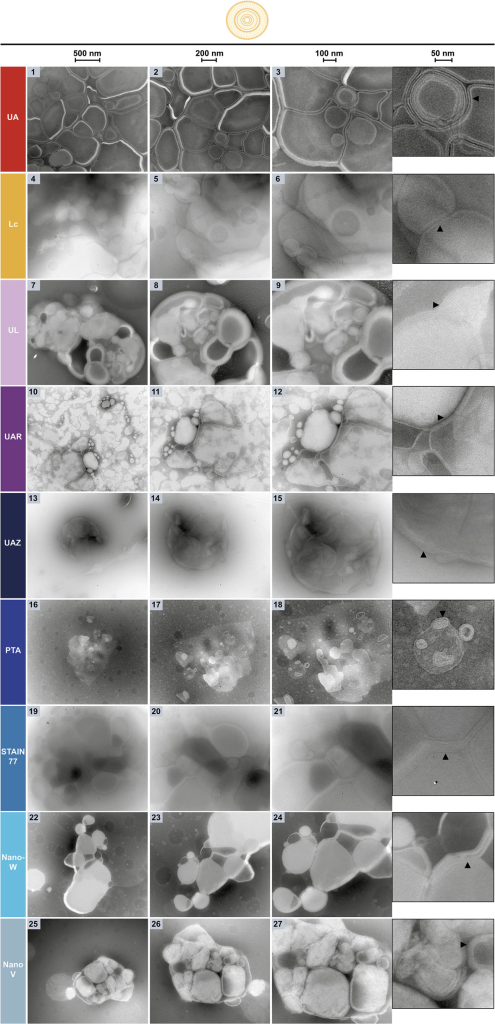
NsTEM of POPC-liposomes on carbon support grids. The three columns and zoom-insets show the sample at increasing magnifications (see
scale bars: 500, 200, 100, 50 nm) with multilamellar liposomes and their individual lipid membranes visible (black arrows).
Selecting the optimal stain for TEM characterization can be challenging due to varying sample requirements. To simplify this process, EMPA developed GUIDE4U, a comprehensive, user-friendly decision-making tool that visually and systematically guides researchers through choosing the most suitable stain for their specific sample types. The tool evaluates critical staining parameters such as resolution, contrast, and preservation of structural integrity, ensuring reliable and reproducible imaging results.
Discover the detailed findings in Advanced Healthcare Materials, Kissling et al., 2025.
EMPA’s Strategic Role within METRINO
These breakthroughs are central to the METRINO project’s mission of developing precise, safe, and standardized metrological methods for innovative nanotherapeutics. EMPA’s research directly supports METRINO’s objectives by:
- Reducing laboratory hazards and environmental impact.
- Enhancing analytical precision for complex biological and nanoscale materials.
- Contributing directly to international regulatory and industrial standardization efforts.
About EMPA
The Swiss Federal Laboratories for Materials Science and Technology (EMPA) is a leading interdisciplinary research institute renowned for its pioneering contributions in materials science and analytical technologies. As a pivotal partner of METRINO, EMPA drives innovation in metrology for healthcare and industrial nanotechnology applications.
Stay tuned for further updates and continued progress from EMPA and the METRINO project!
For more information, or to participate in advancing nanomedicine standards, please contact METRINO.
Follow METRINO on LinkedIn for the latest updates!
The project (22HLT04 MetrINo) has received funding from the European Partnership on Metrology, co-financed from the European Union’s Horizon Europe Research and Innovation Programme and by the Participating States. The authors will ensure that the following meta data is submitted and included for each paper: Funder name: European Partnership on Metrology . Funder ID: 10.13039/100019599. Grant number: 22HLT04 MetrINo